Relating to air inhibition of cyanoacrylates.
 en.wikipedia.org
en.wikipedia.org
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Super Glue Vapour
- Thread starter Arc Tourist
- Start date
prepman
Forum Supporter
- Messages
- 2,770
- Location
- Gwynedd UK
But not cynocrylates.... not mentioned. Silicone adhesive (acetic) also cure with moisture. Cement with Co². Neutral Silicone with? Threadlock is anaerobic and some resins used in laser printing.Relating to air inhibition of cyanoacrylates.
Polymerisation inhibitor - Wikipedia
en.wikipedia.org
Logically the 'baking powder trick' wouldn't work if it was truly anaerobic.
Fixed that for you.But not cynocrylates.... not mentioned. Silicone adhesive (acetic) also cure with moisture. Cement with Co². Neutral Silicone with? Threadlock is anaerobic and some resins used in laser printing.
Logically the 'baking powder trick' wouldn't work if it wastrulysolely anaerobic.
Baking powder is bone dry, so moisture isn't playing a big part, either. Surface chemistry is important in cyanoacrylate polymerisation.
Here are some research abstracts about oxygen and polymerisation:
prepman
Forum Supporter
- Messages
- 2,770
- Location
- Gwynedd UK
Again no mention of cynocrylate being anaerobic. Baking powder is dry but the surrounding air isn't. If it was anaerobic then it wouldn't set ...think about it...slowly....4 most common adhesives for gluing metal to metal and metal to other materials
Anaerobic metal adhesives are used only for metal to metal bonding for applications like threadlocking.
Fixed that for you.
- Epoxy is one of the strongest adhesives for metal. It exists in different types which have unique properties such as chemical resistance and heat resistance. Epoxies come as one or two component systems. Single component epoxy glue for metal cures as a result of additional heat. A 2K system is a mixture of two parts with react with each other starting the curing process. When looking for the strongest glue for metal, a 2K epoxy for metal should be considered.
- Acrylic metal glue exists as two types: surface activated and and bead on bead acrylics. The latter refers to applying a bead of adhesive on both substrates before connecting them. The bonding starts when the parts comne in contact with each other and become subject to pressure. Surface activated acrylics, in turn, require a water thin initiator applied to one substrate and the resin to the other.
- Cyanoacrylate, also known as instant adhesive, is the super glue for metal to metal bonding. Cyanoacrylate adhesives is suitable for most metals as long as they are reactive. Therefore, the super glue for metal works better on brass and copper than on steel. Due to the excellent performance as metal glue, commercial cyanoacrylate adhesives are very popular among miniature and modeling hobbyists.
- Anaerobic metal adhesives are only used for gluing metal to metal as they require presence of metal and absence of oxygen to be able to cure. Anaerobic adhesives are ideal for securing fasteners in terms of threadlocking and thread sealing as well as gasketing and retaining.
Baking powder is bone dry, so moisture isn't playing a big part, either. Surface chemistry is important in cyanoacrylate polymerisation.
Here are some research abstracts about oxygen and polymerisation:
Complete Guide to Metal Adhesive | Metal Glue
Metal adhesives are various and versatile, but which one is the best for your application? Discover the metal glue options per metal type
Prajna
Fixing things for the love of it
- Messages
- 683
- Location
- Castelo Branco, Portugal
Also graphite. Utube superglue, baking soda and graphite.I have only just found out about adding Baking Soda to super glue, very impressed
PhillipM
Member
- Messages
- 3,079
Fixed that for you.
Baking powder is bone dry, so moisture isn't playing a big part, either. Surface chemistry is important in cyanoacrylate polymerisation.
Here are some research abstracts about oxygen and polymerisation:
Baking powder kicks the reaction off because the inhibitor in superglue is slightly acidic. Baking powder neutralises that and kicks off the polymerization. It's also usually that which kicks the reaction off in the case of surface moisture too, rather than the moisture itself. But yes, there's oxygen inhibition going on as well, oxides cap the ends of the chains and massively slow polymerisation. It's why even if you pour out a load of superglue and then stir water in it in the open air it doesn't just set instantly. Close fitting parts - especially active metals, act as catalysts and accelerate things rapidly. It's why superglue cures so much faster in thin bond lines, you exclude a lot of oxygen, you raise the PH more from surface moisture that's alkaline, and you have a large catalytic surface to get the oxide end caps out of there as well.
It's never as simple as one easy, clear mechanism, there's a lot at work.
prepman
Forum Supporter
- Messages
- 2,770
- Location
- Gwynedd UK
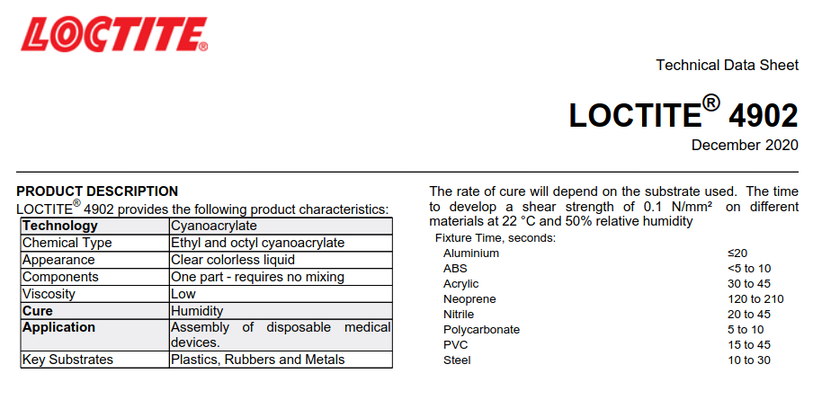
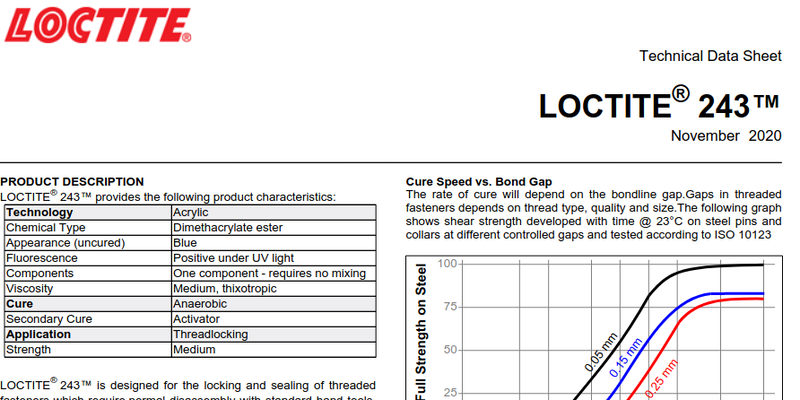
But the short of it is that it is not classed as an anaerobic adhesive. 1st pic. There are distinctive ones that are. i.e Threadlok (acrylic) 2nd pic Please look at the cure column. Mfr is HenkelBaking powder kicks the reaction off because the inhibitor in superglue is slightly acidic. Baking powder neutralises that and kicks off the polymerization. It's also usually that which kicks the reaction off in the case of surface moisture too, rather than the moisture itself. But yes, there's oxygen inhibition going on as well, oxides cap the ends of the chains and massively slow polymerisation. It's why even if you pour out a load of superglue and then stir water in it in the open air it doesn't just set instantly. Close fitting parts - especially active metals, act as catalysts and accelerate things rapidly. It's why superglue cures so much faster in thin bond lines, you exclude a lot of oxygen, you raise the PH more from surface moisture that's alkaline, and you have a large catalytic surface to get the oxide end caps out of there as well.
It's never as simple as one easy, clear mechanism, there's a lot at work.
Products
Last edited:
premmington
Member
- Messages
- 6,558
- Location
- Norfolk
Been following this thread. I never tried glue sniffing as a teenager - what a sheltered life I have had. 

prepman
Forum Supporter
- Messages
- 2,770
- Location
- Gwynedd UK
Never too....sniff...sniff.....late......to...sniff....start?Been following this thread. I never tried glue sniffing as a teenager - what a sheltered life I have had.
But consider this. Superglue will set far faster in an assembled threaded fastener than most, if not all, threadlocking products, yet it doesn't polymerise when applied to one thread or the other (male / female), prior to assembly. So, what is going on there if it isn't anaerobicity?
Cobbler
Codger bodger
- Messages
- 8,089
- Location
- Gloucestershire UK
I find with superglue, it sticks your fingers instantly, most other things it far from instant. Hold it together, wait, blow on it, it seems pot luck whether it’s instant or not, I’ve never tried the accelerator though, is it worth while?
prepman
Forum Supporter
- Messages
- 2,770
- Location
- Gwynedd UK
Sounds like the manufacturers are mistaken?But consider this. Superglue will set far faster in an assembled threaded fastener than most, if not all, threadlocking products, yet it doesn't polymerise when applied to one thread or the other (male / female), prior to assembly. So, what is going on there if it isn't anaerobicity?

No, the manufacturers are not mistaken. Think of it from the manufacturer's perspective though, and the involvement of marketing departments. Superglue is used quite a bit in medical applications (likely to be quite a lucrative side of the business), where water plays a big part in the polymerisation process so, what do they decide to put on the technical data sheet? The TDS is not a full and detailed summary of all the known chemistry (which they might not want to reveal too much about in any case). The fact is, water, oxygen, surface chemistry, and the inhibitors incorporated in the glue mixture, all play a part.
prepman
Forum Supporter
- Messages
- 2,770
- Location
- Gwynedd UK
Pass.No, the manufacturers are not mistaken. Think of it from the manufacturer's perspective though, and the involvement of marketing departments. Superglue is used quite a bit in medical applications (likely to be quite a lucrative side of the business), where water plays a big part in the polymerisation process so, what do they decide to put on the technical data sheet? The TDS is not a full and detailed summary of all the known chemistry (which they might not want to reveal too much about in any case). The fact is, water, oxygen, surface chemistry, and the inhibitors incorporated in the glue mixture, all play a part.
PhillipM
Member
- Messages
- 3,079
But the short of it is that it is not classed as an anaerobic adhesive. 1st pic. There are distinctive ones that are. i.e Threadlok (acrylic) 2nd pic Please look at the cure column. Mfr is Henkel
Products
www.henkel-adhesives.com
Because as I said, there's far more than one mechanism at play. Anerobic cyanacrylate threadlockers won't set on many materials, even with tight fitting threads and no oxygen around.
If they're purely anerobic like the TDS says, they should cure. But they don't, because they need other conditions *as well* to catalyse and kick off the reaction.
You can kick off superglue with no moisture at all - mix some baking powder in a big tub of superglue and stand back as it'll cure so damned fast it'll probably get up to a few hundred degrees, smoke like hell and probably give you serious eye pain if you're anywhere near it - no moisture required at all.
Same with adding purified, PH neutral water. It won't actually kick off the glue because it won't neutralise the inhibitor in the mix.
prepman
Forum Supporter
- Messages
- 2,770
- Location
- Gwynedd UK
PassBecause as I said, there's far more than one mechanism at play. Anerobic cyanacrylate threadlockers won't set on many materials, even with tight fitting threads and no oxygen around.
If they're purely anerobic like the TDS says, they should cure. But they don't, because they need other conditions *as well* to catalyse and kick off the reaction.
You can kick off superglue with no moisture at all - mix some baking powder in a big tub of superglue and stand back as it'll cure so damned fast it'll probably get up to a few hundred degrees, smoke like hell and probably give you serious eye pain if you're anywhere near it - no moisture required at all.
Same with adding purified, PH neutral water. It won't actually kick off the glue because it won't neutralise the inhibitor in the mix.
prepman
Forum Supporter
- Messages
- 2,770
- Location
- Gwynedd UK
Moist fingers?I find with superglue, it sticks your fingers instantly, most other things it far from instant. Hold it together, wait, blow on it, it seems pot luck whether it’s instant or not, I’ve never tried the accelerator though, is it worth while?
prepman
Forum Supporter
- Messages
- 2,770
- Location
- Gwynedd UK
What irony....just repaired sliders on a rear caliper and got my new bottle of Threadlok 243 for bolts
 . It wouldn't pour. Congealed mass in bottom of bottle. I looked closely at bottle for 1st time.
. It wouldn't pour. Congealed mass in bottom of bottle. I looked closely at bottle for 1st time.
The balloons gone up now!
Went back to eBay listing.....I should have read it more carefully. Speed reading is a no-no.
Buyer beware!

 . It wouldn't pour. Congealed mass in bottom of bottle. I looked closely at bottle for 1st time.
. It wouldn't pour. Congealed mass in bottom of bottle. I looked closely at bottle for 1st time.The balloons gone up now!
Went back to eBay listing.....I should have read it more carefully. Speed reading is a no-no.
Buyer beware!